Want to see the full answer. 2 millimolar is 16 gL using the 8000 MW - that seems reasonable.

How To Calculate Molarity Using Solute Mass Chemistry Study Com
Volume expressed in either liters milliliters or microliters.

. Textbook solution for CENTRAL SCIENCE-ACCESS CARD 17th Edition Brown Chapter 45 Problem 4112PE. Expertise on every level to craft science technology solutions in life science. Boiling point elevation and freezing point depression.
This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a solution. The correct option is a. Molarity No of molesVolume.
This is the currently selected item. Now if 98 grams of sulphuric acid is present in 1 litre of the solution then it is 1 molar solution of Sulphuric acid. C is the molar concentration in molL Molar or M.
Determine the molarity of a solution made by dissolving 0323 moles of ethanol in enough water to make 5000 mL of solution. How does this molarity calculator work. If youre seeing this message it means were having trouble loading external resources on our website.
A 150 M B 118 M C 0130 M D 0768M E 230 M 29 Identify ammonia A strong electrolyte strong base B strong electrolyte weak base C. 5 rows The following equation allows you to find the molarity of a solution. The Molecular mass of Sulphuric acid is 98.
N number of moles of urea 12060 2 mol. Representing solutions using particulate models. Mass g Concentration molL x Volume L x Molecular Weight gmol An example of a molarity calculation using the Tocris molarity calculator.
Expert Solution Answer. What is the molarity of a solution of 6 grams of NaCl 1 teaspoon of table salt dissolved in 500 milliliters of water. M M moles liter.
Molarity concentration. To find the molarity of a solution we divide the number of moles of solute by the total volume of liters of solution. We have step-by-step solutions for your textbooks written by Bartleby experts.
Molar concentration also known as molarity and can be denoted by the unit M molar. 2 millimolar in monomer MW 72 is only 014 gL which is pretty dilute. But it depends on.
Practice calculations for molar concentration and mass of solute. To prepare 1 L of 05 M sodium chloride solution then as per the formula use 2922 g of sodium chloride 05 molL 1L 5844 gmol 2922 g. Check out a sample textbook solution.
Moles solute per liter of solution not volume of solvent added since the solute takes up some space symbol. Molarity moles of solute litres of solution. Molarity is defined as the number of moles of solute dissolved per liter of solution molL M.
The Tocris molarity calculator is based on the following equation. Determine the molarity of a solution made by dissolving 261 moles of potassium nitrate in enough water to make 5000. When the amount of solute is given in grams then we must first find or calculate the number of moles of solute.
Moles of Ethanol 004 Mol. Calculate the molarity of the following solutions a 4 mathrmg of caustic soda is dissolved in 200 mathrmmL of the solution mathrm. V volume of the solution mass of solution density of solution 120 1000 g 115 g mL-1 974 mL 0974 L.
Molarity of a solution M nV. The molarity of the given solution. Molecular weight expressed in gmol.
Molar solution concentration equation. To find the number of moles of solute we can calculate by dividing. The molarity of this solution is.
Determine the molarity of a solution made by dissolving 453 g of ethanol C 2 H 5 OH in enough water to make 15000 mL of solution. Mass expressed in either grams milligrams micrograms. The molarity calculator equation.
As per the given details. Ad Find this and more substances to complete your research. And therefore molarity will be 1.
Its one of the easiest units to calculate. Concentration expressed in either. 28 Determine the molarity of a solution formed by dissolving 977 g LiBr in enough water to yield 7500 mL of solution.

Molarity Chemistry Tutorial Youtube
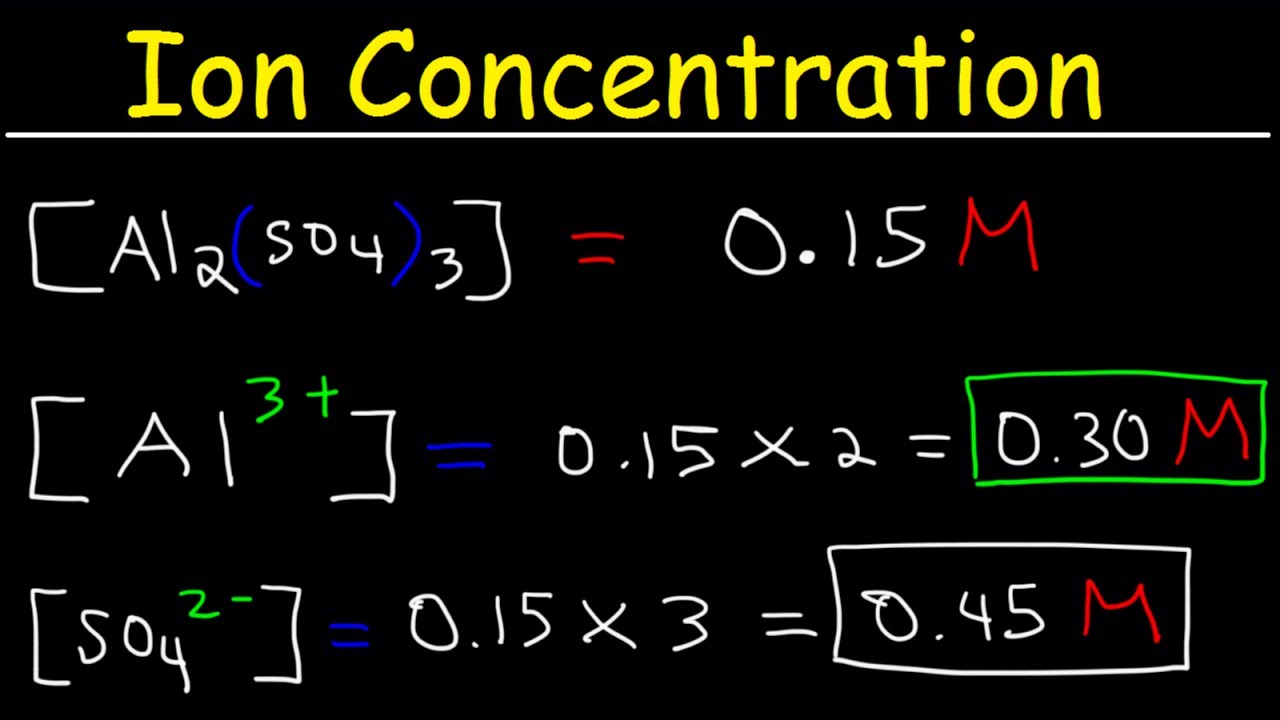
Molarity Practice Problems Youtube

Question Video Calculating The Molarity Of A Solution From Mass And Volume Nagwa

0 Comments